Improving wrist injury pathways by developing a complex intervention
By Benjamin Deana, Chris Littleb, Ashley Scrimshirec, Amy Groved and Tim Stephense
aConsultant Hand Surgeon and Senior Research Fellow, Oxford University Hospitals NHS Trust
bConsultant Hand Surgeon and Senior Research Fellow, Oxford University Hospitals NHS Trust
cOrthopaedic Registrar and PhD Student, University of York
dAssociate Professor, University of Warwick
eQuality Improvement Specialist, Queen Mary, University of London and Barts NHS Trust
Published 21 July 2022
Introduction
Wrist injuries represent a significant burden to both patients and health care services in the UK. Around 70,000 patients per year attend NHS Emergency Departments (EDs) or Minor Injuries Units (MIUs) present after trauma with significant wrist pain and tenderness on clinical examination, and then go on to have entirely normal x-rays; these patients are often described as having ‘suspected scaphoid fractures’ or ‘occult’. Current guidance from the National Institute of Care and Health Excellence (NICE) for ‘suspected scaphoid fractures’ advises early magnetic resonance imaging (MRI), with ‘early’ in this context being imaging requested directly from the first clinical encounter.
So, what is the problem?
In order to provide the best treatment for all patients, we, as clinicians need an investigation which can reliably detect and exclude scaphoid fractures. MRI has been consistently proven to be the most sensitive investigation for detecting scaphoid fractures, which means it is unique in being able to confidently rule out the presence of a scaphoid fracture and enable early mobilisation in a majority of patients1,2. The superiority of early MRI translates into an improved patient experience and significantly lower healthcare costs3-5. This was evidenced in the Scaphoid Magnetic Resonance Imaging in Trauma (SMaRT) trial which compared early MRI to a more traditional pathway of further assessment combined with delayed CT when clinically indicated. Trial authors reported substantial cost savings (£266 per patient at six months), as well as benefits in terms of patient satisfaction and experience3.
Evidence suggests that x-rays fail to detect around 40% of scaphoid fractures, while computerised tomography (CT) fails to detect around 30% of occult scaphoid fractures6,7. The inferior sensitivity of x-ray and CT based pathways mean that clinicians cannot confidently enable patients with pain to get their wrists moving early with negative imaging findings. Even with prompt cast immobilisation, the outcome of an undisplaced occult scaphoid fracture is not necessarily benign and may result in delayed union6. The rate of delayed union in undisplaced fractures is around 6% and this can only be increased by a failure to diagnose and treat the injury promptly with immobilisation8. There are also significant consequences in terms of litigation for failing to promptly treat fractures which go onto develop complications, such as delayed union9.
Despite the evidence for early use of MRI scans and supporting NICE guidance, we know from our recent national survey that Wrist Injury Pathways (WIPs) in the UK are highly variable10. We found a total of 66% (57 of 87 centres) of UK centres routinely use MRI in their pathway. However, only a minority (14%, 12 centres) offered MRI directly from ED/MIU and 52% (45 centres) offered delayed MRI after repeating clinical examination in either an ED or specialist clinic. This implementation gap between what best evidence recommends and what happens in clinical practice is a complex issue that requires further investigation.
Why the need for a complex intervention?
Clinical interventions in surgery exist on a spectrum from simple, such as closing a wound with stitches, to the complicated (carrying out a joint replacement for a neck of femur fracture) to complex (improving trauma theatre efficiency). The latter, more complex variety, are characterised by 1) the number of components in the intervention; 2) the range of behaviours targeted; 3) the range and different levels of target recipients; 4) the expertise and skills required by those delivering and receiving the intervention; and 5) or by the level of flexibility permitted in the intervention delivery. We have already demonstrated a low rate of compliance with NICE guideline number 38 (NG38) over the five years since its publication, which reveal clear failure to implement recommendations10. Our national survey revealed that implementation failure required more than just MRI capacity. To explore these issues further, our team organised an expert stakeholder event at the British Hand Society’s national meeting in 2021. Discussions revealed multiple potential implementation barriers:
- MRI capacity
- Lack of awareness of the evidence and guidance amongst all stakeholders
- Lack of agreement on the need for service change amongst all stakeholders
- A lack of integration of services
- A deficiency of clear pathways of care
- Inadequate staffing resource to manage the integrated pathway
- A paucity of efficient data gathering
Understanding and overcoming this variability and complexity need to be tackled with equally complex and variable methods. Addressing the problem effectively will require an understanding of the extent and impact of the issues we identified across a variety of hospitals. This real-world evidence needs to feed into the development of interventions that is sufficiently flexible to the variable contexts in which they are applied.
The National Institute for Health and Care Research (NIHR) and the MRC have recently updated the guidance on developing complex interventions to include a stronger emphasis and recognition of the complexity of systems into which complex interventions are first tested, and then put into practice11. This guidance advises that six core elements are considered when developing a complex intervention and important for our future work in closing the implementation gap in the wrist injury pathway. These core elements include considering the context to the intervention, the diverse views of stakeholders, the key uncertainties and the development of theory as to how the intervention may work.
Important aspects of developing a complex intervention to address this problem
Carol Weiss, a leading American scholar on policy analysis coined the phrase ‘there is nothing as practical as good theory’12. This viewpoint has long been held by designers and evaluators of complex social and community interventions. Yet, the clinicians and managers tasked with designing complex interventions in the hospital setting appear to be behind the curve. Complexity in healthcare necessitates the identifying and development of appropriate programme theory to support intervention development, as specified by the NIHR/MRC’s second core element above13. Developing clear theory to support an intervention can help to justify what changes are required, how change is to be achieved and crucially why we think our change interventions will work. A vital early task in ensuring evidence-based treatment of suspected scaphoid fracture is to develop a theoretical understanding of the likely process of change to understand the drivers (or root causes) of the problem. To do this, we need to draw on existing evidence and theory from similar contexts, and verify our initial assumptions through primary research, for example interviews with stakeholders and the detailed mapping of care pathways. In this case, the relevant stakeholders are a highly diverse group including commissioners, managers, ED staff, administrative staff, radiologists, and surgeons.
It will be important to consider the type of potential barriers and facilitators to complex interventions in orthopaedic surgery. The Donabedian model of quality assessment is useful here14: for any given outcome (in our case early MRI of the wrist) is the product of process factors (the way care is organised or delivered) and structural factors (the physical capacity of the service to deliver the target outcome). Our exploratory work has demonstrated deeply entrenched negative or apathetic views regarding the value of early MRI are unlikely to be a barrier to change. This is positive as it suggests that most centres would be amenable to changing local processes. However, MRI capacity is likely to be a much more significant barrier to implementation. Even so, this can still be considered using the process vs. structural lens. Process barriers would include inefficient use of existing MRI capacity, which would be amenable to pathway implementation to streamline and improve efficiency. Conversely structural problems, such as insufficient capacity despite optimising MRI efficiency may require the purchase of more scanners and recruitment of more staff etc. Structural problems are harder to influence with behaviour change interventions and generally need a different approach e.g., business case, funding etc15.
Developing a theory of change can involve the generation of driver diagrams. A driver diagram is a simple graphic representation, consisting of the project’s ‘goal’ and the factors, i.e., the drivers, which need to be influenced in order to achieve the goal (Figure 1).
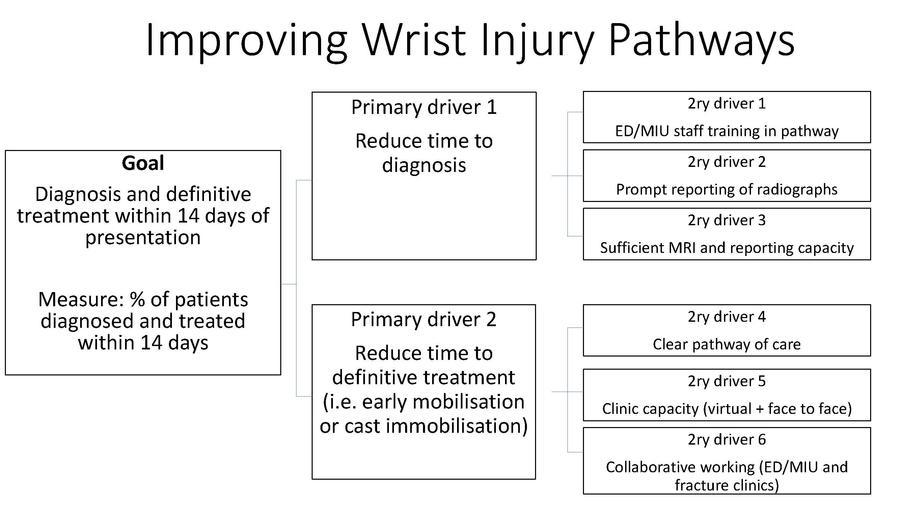
We have previously identified many of the barriers which limit implementation and set us on track to understanding the drivers16. The goal of our work, as supported by the evidence and guidance, is ‘simply’ early access to MRI and the prompt actioning on the results of MRI. A theory of change is a more comprehensive description and illustration of how and why a desired change is expected to happen in a specific context and any necessary preconditions for this (Figure 2).
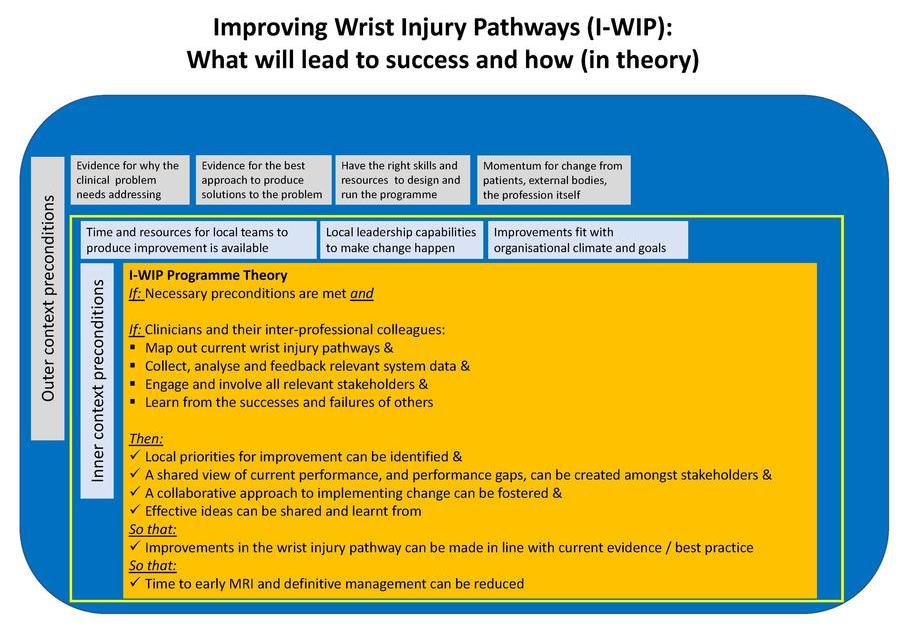
A key aspect of delivering any complex intervention is implementation outcome and process data monitoring and therefore, data systems are an important aspect of intervention development that need to be considered. Data collection systems should be as easy to use and parsimonious as possible e.g., an easy to use data platform which can feedback performance in real time can be a significant facilitator. Those working in the NHS will know that most of our current systems are a far cry from the ideal. Finally, there is a degree of complexity in this case in terms of both the people involved and the setting, as these injuries are managed in many different settings and frequently in a way that involves several different departments within a hospital. To evaluate the effect of a complex intervention within a complex system, we need to model expected change through early feasibility work, to assess if it is possible to achieve implementation goals and then, if so, formally pilot the complex intervention in a range of settings before moving to a larger trial.
The way forward
Further work is clearly required to further understand the complexity in the system. We believe that the core elements to this will include developing the programme theory and the supporting driver diagrams, while ensuring that diverse stakeholder perspectives are included within our future work. This will require mixed methods research designs including qualitative interviews and pathway mapping at multiple centres across the UK. Over time with the input of both intervention experts and a diverse group of stakeholders we envision that a complex intervention can take shape, with the eventual aim of testing the effectiveness of this intervention in the context of the NHS.
The need for change to improve wrist injury pathways is clear, and for a complex intervention to have any credible chance of being effective, it is essential that the development process is undertaken in a evidence informed, thoughtful, and ultimately scientific manner.
References
- Bäcker HC, Wu CH, Strauch RJ. Systematic Review of Diagnosis of Clinically Suspected Scaphoid Fractures. J Wrist Surg. 2020;9(1):81-9.
- Yin ZG, Zhang JB, Kan SL, Wang XG. Diagnosing suspected scaphoid fractures: a systematic review and meta-analysis. Clin Orthop Res Rel. 2010;468(3):723-34.
- Rua T, Malhotra B, Vijayanathan S, Hunter L, Peacock J, Shearer J, et al. Clinical and cost implications of using immediate MRI in the management of patients with a suspected scaphoid fracture and negative radiographs results from the SMaRT trial. Bone Joint J. 2019;101-B(8):984-94.
- National Clinical Guideline Centre (UK). Fractures (Non-Complex): Assessment and Management. London: National Institute for Health and Care Excellence (NICE); 2016 Feb. (NICE Guideline, No. 38.) Appendix M, Cost-effectiveness analysis: Imaging of suspected scaphoid fractures. Available from: www.ncbi.nlm.nih.gov/books/NBK368138.
- Patel NK, Davies N, Mirza Z, Watson M. Cost and clinical effectiveness of MRI in occult scaphoid fractures: a randomised controlled trial. Emerg Med J. 2013;30(3):202-7.
- Dean BJF, Riley N, Little C, Sellon E, Sheehan W, Burford J, et al. Suspected scaphoid injuries managed by MRI direct from the ED – a single centre prospective cohort study. Bone Jt Open. 2021;2(6):447-53.
- Sahu A, Kuek DK, MacCormick A, Gozzard C, Ninan T, Fullilove S, et al. Prospective comparison of magnetic resonance imaging and computed tomography in diagnosing occult scaphoid fractures. Acta Radiol. 2021 Dec;2841851211064595.
- Dias JJ, Brealey SD, Fairhurst C, Amirfeyz R, Bhowal B, Blewitt N. Surgery versus cast immobilisation for adults with a bicortical fracture of the scaphoid waist (SWIFFT): a pragmatic, multicentre, open-label, randomised superiority trial. Lancet. 2020;396(10248):390-401.
- Harrison W, Newton AW, Cheung G. The litigation cost of negligent scaphoid fracture management. Eur J Emerg Med. 2015;22(2):142-3.
- Dean BJF. The management of suspected scaphoid fractures in the UK: a national cross-sectional study. Bone Jt Open. 2021;2(11):997-1003.
- Skivington K , Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061.
- Weiss C, (1995). Nothing as Practical as Good Theory: Exploring Theory-Based Evaluation for Comprehensive Community Initiatives for Children and Families. In: New Approaches to Evaluating Community Initiatives: Concepts, Methods, and Contexts, The Aspen Institute, 65-92.
- May C. Towards a general theory of implementation. Implement Sci. 2013;8(1):18.
- Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83(4):691-729.
- Stephens TJ, Beckingham IJ, Bamber JR, Peden CJ. What Influences the Effectiveness of Quality Improvement in Perioperative Care: Learning From Large Multicenter Studies in Emergency General Surgery? Anesth Analg. 2022;134(3):559-63.
- Peden CJ, Stephens T, Martin G, Kahan BC, Thomson A, Rivett K, et al. Effectiveness of a national quality improvement programme to improve survival after emergency abdominal surgery (EPOCH): a stepped-wedge cluster-randomised trial. Lancet. 2019;393(10187):2213-21.
