Evidence based suggestions for the return to elective orthopaedic surgery following the COVID-19 pandemic
By Sarkhell Radhaa,b and Irrum Afzalb
aCroydon University Hospital London, UK
bSouth West London Elective Orthopaedic Centre, UK
Corresponding author: Mr Sarkhell S Radha MBChB, PG Cert, MSc, MBA, MRCS, FRCS (Tr & Orth)
Consultant Trauma and Orthopaedic Surgeon
COVID-19 Strategy Lead and Business Continuity Manager
COVID-19 Coordinating Consultant
Email: s[email protected]
Published 01 May 2020
Editor’s note: They say that nature abhors a vacuum so as participants in the natural world we are as guilty as others in wanting to fill one. There is a void of guidance waiting to be filled. We know that for some things we are better given a free hand to act locally but that for others we need to work as a pack, as this provides consistency, resilience, safety, reproducibility and all the other products of unified standardised working which we have been advocating as a profession. The environment in which we are restarting elective practice in the endemic phase of COVID-19 is currently “An Official Guidance Void” that needs to be filled.
The TJTO&C was aimed at sharing and reflecting opinion and so it is quite appropriate to publish members' views on the current situation even while we wait for a central steer. So, as with other articles it represents the authors' opinions and not the BOA’s policy views on how to proceed. The BOA is pressing hard for guidance but otherwise are keeping our powder dry until we know the rules within which we will have to function.
Abstract
The COVID-19 pandemic has presented the world with increased challenges. Despite immediate mortality and morbidity following this crisis, long-term implication on healthcare service provision has not been clearly evidenced in the literature. At present, there are currently no approved, nor proven, effective treatments or vaccines available for COVID-19.
With this in mind, one can speculate COVID-19 can stay for a considerable period of time, worldwide, with health and economic consequences. COVID-19 has led to a severe overloading of hospital systems in many affected regions and countries. Healthcare resources have been rearranged to manage the influx of a large number of patients requiring intensive monitoring, artificial ventilation, and in selected cases extracorporeal membrane oxygenation (ECMO). The response to this pandemic has led to a sudden disruption of routine medical and elective surgical care. In an attempt to conserve resources and slow the spread of disease the NHS England and British Orthopaedic Association (BOA) ceased all elective service to train and re-deploy their staff to support the increased pressures from COVID-19. The temporary halt on elective procedures has an impact on the financial integrity of healthcare systems that are disproportionately dependent on elective procedures as a source of revenue. In the foreseeable future, it is important for hospitals and healthcare providers to reinstate elective services.
We present evidence-based guidance to allow a phased return of the much needed elective orthopaedic service. The return pathway presented here could potentially be used as a model for other surgical specialities.
Introduction
In late December 2019, cases of acute respiratory diseases were reported in Wuhan, Hubei province, China. As the causative agent for these cases was unable to be identified, initial cases were classified as "pneumonia of unknown etiology”1. After intensive outbreak investigations, by the Chinese Centre of Disease Control and Prevention, the etiology of this illness was attributed to a novel virus belonging to the coronavirus (CoV) family. In the past twenty years, several viral epidemics such as H1N1 influenza, Acute Respiratory Syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV have occurred2.
The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) associated disease (COVID-19) has rapidly escalated to pandemic proportions as declared by WHO on 11th March 20203-5. To date over 2,936,427 cases of confirmed infections and over 200,000 deaths from COVID-19 have been reported worldwide6.
There have been increased cases of COVID-19 reported in patients with comorbidities such as respiratory, cardiovascular disease, males and smokers7-9. At present, there are currently no approved, nor proven effective treatments or vaccines available for COVID-19. There are several on going trials of potential therapies including immunisations10,11.
With this in mind, one can speculate COVID-19 can stay for a considerable long period of time worldwide with health and economic consequences. COVID-19 has led to a severe overloading of hospital systems in most affected regions and countries12. Healthcare resources have been rearranged to manage the influx of a large number of patients requiring intensive monitoring, artificial ventilation, and in selected cases extracorporeal membrane oxygenation (ECMO)13.
Even in countries where COVID-19 has not attained high incidence rates, containment measures are recommended and are being implemented, to prevent infections of both patients and healthcare professionals14.
The response to this pandemic has led to a sudden disruption of routine medical and elective surgical care. Most hospitals across the globe have adapted virtual review for imminent elective clinics and have postponed almost all elective surgical procedures. In an attempt to conserve resources and slow the spread of disease the NHS England and British Orthopaedic Association (BOA) ceased all elective surgeries to train and re-deploy their staff to support the increased pressures from COVID-1915,16. The temporary halt on elective procedures has an impact on the financial integrity of healthcare systems that are disproportionately dependent on elective procedures as a source of revenue18-20.
The aim of this paper is to present evidence based guidance to allow a phased return of the much-needed elective orthopaedic service. The return pathway presented here could potentially be used as a model for other surgical specialities.
Phased return of elective work
During the COVID-19 crisis, most elective orthopaedics procedures have been deemed a non-essential procedure to help conserve resources for COVID-19 patients25,26. Resuming various elective procedures including Total Joint Arthroplasty (TJA) will also be essential to improve the well-being of our patients27. However, it must be done in a safe and sustainable manner. When resuming elective surgery, healthcare providers should monitor their local and national COVID-19 profile. Assessing the availability of COVID-19 testing and setting testing policies have been suggested as an imperative step in resuming elective surgical procedures. Bringing back elective orthopaedic surgery would need staff such as anaesthetists to be taken off their COVID-19 duties and equipment such as ventilators being used to support the COVID-19 to be repurposed back to elective orthopaedic theatre facilities25.
Here we present guidance for the resumption of required elective orthopaedic surgery. If properly implemented, we anticipate a return to a full service within six months to nine months.
The current COVID-19 profile in the United Kingdom
The estimated peak of cases was on 7th April 2020 (Fig 1). Since, then there has been a fluctuation in the number of cases diagnosed in the UK. The number of deaths from COVID-19 has shown a steady decline from 11th April 2020 (Fig 2). Although, the decline in diagnosed cases and mortality rate may be due to the result of government's stringent preventative measures, it does provide indication that a further decline is expected within the coming weeks. It is therefore imperative that processes are implemented to reinstate elective orthopaedics work.
In agreement with published literature, prior to the start of any elective work, there should be a sustained reduction in the rate of new COVID-19 cases and the number of deaths for 14 days22-24. It is important there is no compromise to the number of Intensive Care Unit (ICU) and personal protective equipment (PPE), ventilators when restarting elective orthopaedic work22-24.
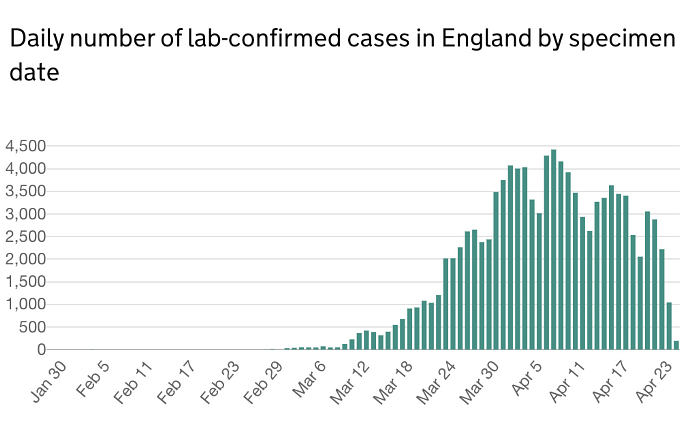
Figure 1. Illustrates the number dial number of lab confirmed cases in England by specimen date.
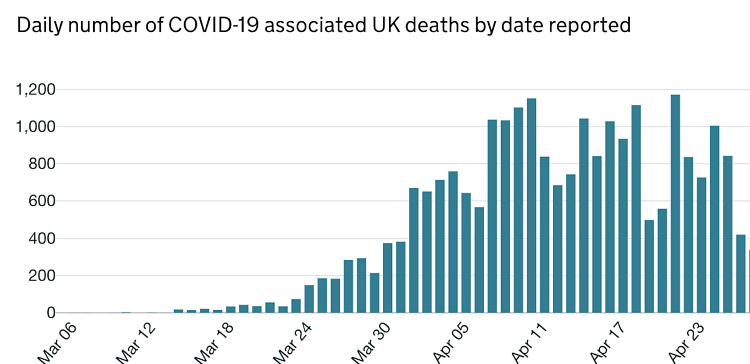
Figure 2. Illustrates the number of COVID-19 pandemic deaths.
In preparation for resuming elective orthopaedic service
Analysis of local COVID-19 data
- Identify and analyse data from COVID-19 admissions into the hospital (local COVID-19 profile). Patient analysis must include: patient demographics, patient comorbidity, intensive care admission, high dependency unit admission, mortality rates and the number of patients discharged.
- Gather list of patients who have their planned surgery cancelled due to COVID-19 pandemic or pending elective procedures, which have not yet been listed. The list of patients should include the patient's demographics, patients’ social status, patients’ post-operative care requirements and the level of support required and current COVID-19 status. Extra caution should be taken into consideration for patients who reside in care homes or nursing homes or will require nursing home care post- operatively. If the situation allows, these cases should be undertaken following the resolution of the pandemic27-30.
- Classify the current COVID-19 patients into three groups, low risk, moderate risk and high risk depending on their symptoms, test results and local COVID-19 profile31.
- Match the outstanding elective patients profile to COVID-19 groups, i.e. risk stratify these patients into low risk, moderate, and high risk compared with the COVID-19 patient profile.
- Classify the elective orthopaedic procedures into various categories: day case surgery, short in patients’ stay and long inpatients stay plus or minus High Dependency Unit requirements following the planned procedure.
Analysis of resource requirements for the hospital and the elective orthopaedics
- Implement daily virtual meetings with surgeons, anaesthetist, theatre staff and management staff to discuss case mix, theatre utilisation, theatre efficiency, post-operative care and discharge planning.
- Have a process to deep clean operating theatres daily.
- Ensure availability of appropriate PPE, anaesthetic equipment, implants and medications.
- Establish a dedicated surgical and medical workforce with appropriate training of proper hygiene includes frequent hand washing32.
- Create multiple teams that are completely insulated from each other to ensure there is no cross contamination33.
- All staff to be tested for COVID-19 prior to resumption of elective work. Ensure there is a process and facility for re-testing staff should they become symptomatic. Following the American Academy of Orthopaedic Surgeons (AAOS) guidance, all staff should be given a detection test using Reverse Transcriptase PCR (RT-PCR): Detects SARS CoV-2 viral RNA (ANTIGEN) in oropharynx and nasopharynx: Highly sensitive. At present, this is the best available testing solution, but there are a number of factors to be considered which are not within the scope of this guidance. When available, staff should be offered Antibody testing34.
Process for preoperative assessment:
- Implement a preoperative questionnaire undertaken by an appropriate health care professional using telemedicine.
- All patients should complete a questionnaire 14 days prior to surgery with appropriate healthcare professionals using telemedicine. This will allow us to optimize patient selection. In addition, this 14 day window will allow for additional testing or imaging required for preoperative planning.
- Any laboratory testing and radiologic imaging procedures should be determined over the phone. Testing and repeat testing without indication is discouraged.
- Any laboratory testing and radiologic imaging required will be taken five days prior to surgery.
- Methicillin-resistant Staphylococcus aureus (MRSA) and Methicillin-sensitive Staphylococcus aureus (MSSA) swabbing will be done five days prior to surgery. All patients regardless of test results will be given Octenisan body wash at their five day visit. They are advised to use this either four days prior to surgery and one day after surgery, or three days prior to surgery and 2 days after surgery36-39.
- All patients will be provided with postoperative guidance through a dedicated musculoskeletal therapist or occupational therapist. The hospital should work on provision of leaflets and/or video links to aid postoperative rehabilitation.
- Assessment for the need for post-operative care should be undertaken in the preoperative questionnaire.
COVID-19 screening
- In addition to the regular pre-operative screening questionnaires, patients will be invited for COVID-19 swabbing five days prior to surgery.
- Any face to face pre operative assessment and COVID-19 test and or swabbing required will be undertaken in one sitting at the hospital five days prior to the planned surgery.
- The COVID-19 screening questionnaire will then be repeated again 48 hours before surgery. The incubation period for COVID-19, which is the time between exposure to the virus (becoming infected) and symptom onset, is on average 5-6 days, however can be up to 14 days. These estimates of the incubation period of COVID-19 are also in line with those of other known human coronaviruses, including SARS (mean, 5 days; range, 2 to 14 days. MERS (mean, 5 to 7 days; range, 2 to 14 days and non-SARS human coronavirus mean, 3 days; range, 2 to 5 days40-43.
- Questions asked in this COVID-19 screening questionnaire will allow medical professions to identify if the planned procedure can go ahead (Table 1).
- The Journal Bone and Joint Surgery listed the clinical symptoms in 3,470 patients with COVID-19, the list of symptoms presented are fever (83%), cough (61%), fatigue (27%), sputum production (21%), muscle aches (14%), gastrointestinal symptoms (10%), Dyspnoea (12%), sore throat (8%), headache (9%), and upper airway symptoms (5%)33.
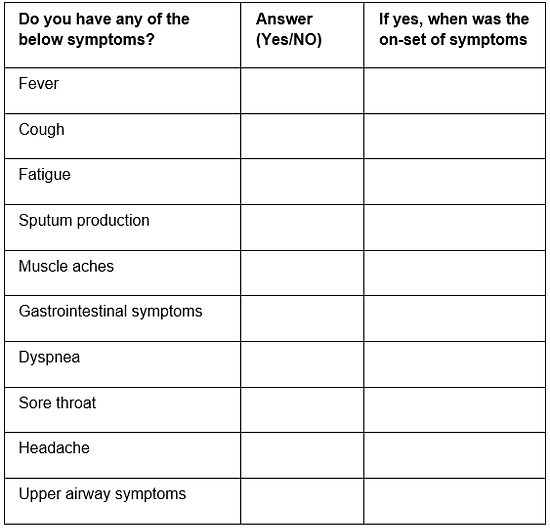
Table 1: COVID-19 screening questionnaire
Analysis of three-phase return to elective surgery
We propose a three-phase strategy for resuming elective orthopaedics surgeries. Prior to returning to elective work, the main priorities are:
- Patient safety and healthcare work safety44,45. In contrast to the literature, we stress the equal importance of both healthcare workers and patients safety during the pandemic. It is important all staff feel safe and well protected when coming into work to undertake elective orthopaedic surgery46-49. Healthcare work professional’s safety is pivotal for effective and efficient patient care.
- Testing of all staff involved in reinstating elective service.
- Adequate multidisciplinary staffing for all elective orthopaedic cases.
- Strong coordination of key staff members, including surgeons, anaesthetist, nurses, and housekeeping.
- Implement a contingency plan in place for staff members who test positive for the coronavirus and staff who have to self- isolate.
- Assess the level of stress and fatigue among healthcare workers who have been providing frontline care during surges of COVID-19 patients.
- Consider mitigation efforts for workforce shortages such as enlisting appropriately trained physician associates or scrub nurses to work as first assistants.
- Clean theatre immediately after every case consider deep cleaning theatre everyday.
Phases of return to elective services
Phase I
- Phase l should be targeted to the patients who will have maximum benefit and those who require urgency in benefit and need for the surgery.
Need = Who stands to have the most significant long-term quality of life (QOL) impairment from not undergoing elective surgery33,34,50.
Benefit = Who stands to have most significant long-term quality of life (QOL) improvement from undergoing elective surgery34,50. - Phase I should be aimed at the low risk of COVID-19 patients. This will be based on the analysis and matching against local hospital COVID-19 profile as well as national and international data, if appropriate.
- No patients who are high risk in developing complications from COVID-19 will be incorporated.
- Case mix will include: day case orthopaedics procedures including day case arthroplasty and ambulatory cases.
- All patients should be x-rayed postoperatively at the same visit, if indicated.
- Patients should receive a 6-week virtual clinic review for follow up.
- At the 6 week time-point, patients will receive Patients Reported Outcome Measures (PROMs) and Patient Reported Experience Measures (PREMs) questionnaires to assess outcome and service satisfaction.
Phase II
- Phase II should be targeted to patients who are matched to low to medium risk.
- Case mix should include short inpatient stay.
- All cases should be pre-rehabbed and guided so they will have an early discharge.
- Phase II should be a priority for patients who may gain the most significant long term QOL impairment from not having surgery and those who might gain most significant long term QOL improvement from undergoing elective surgery.
- All patients should be x-rayed prior to discharge on the same visit, if indicated.
- Patients should receive a 6-week virtual clinic review for follow up.
- At the 6 week time-point, patients will receive PROMs and PREMs questionnaires to assess outcome and service satisfaction.
Phase III:
- Phase III should focus on returning to all elective orthopaedics procedures.
- Case mix should include all elective orthopaedic cases including complex and revision arthroplasty.
- Patient requiring HDU/ITU facility postoperatively will also be operated on providing there is availability of such facility.
- Patients should be encouraged to do early mobilisation and early discharge.
- All patients should be x-rayed prior to discharge during the same visit, if indicated.
- Patients should receive a 6-week virtual clinic review for follow up.
- At the 6 week time-point, patients will receive PROMs and PREMs questionnaires to assess outcome and service satisfaction.
Post-operative guidelines:
- When indicated, all patients from Phase I, II, III should be x-rayed prior to discharge.
- All patients from Phase I, II, III should receive a 6-week virtual clinic review follow up. If the situation improves then the return to face to face clinic should be reinstated.
- All patients from Phase I, II, III should receive a 6-week PROMs and PREMs questionnaires to assess outcome and service satisfaction.
- At any stage, if the patient has any problems, a virtual follow up should be organised. If the problems warrant a face to face consultation, face to face consultation should be organised with no-delay. Normal protocols for emergency and infections should be followed. The patient should not be left at risk despite the COVID-19 pandemic.
General precautions guidelines:
- When possible regional anaesthetic should be deployed in all planned elective cases.
- If the pandemic situation escalates at any stage, temporary halt of elective service should be reinstated.
- Reinstatement of the elective surgical service will also include availability of face to face clinic when appropriate.
- Medical optimisation of patients, preoperative planning and multidisciplinary (MDT) decision making in complex cases, should be undertaken to ensure no compromise is made on provision of the best quality of care.
- Although grading patients according to American Society of Anaesthesiologist (ASA) is of value in all cases, other factors affect outcome following COVID-19 infection which are not covered using ASA grade. We therefore recommend taking into account patients' COVID-19 profile when assessing patients for surgical procedures.
- Further international or national guidance on adding COVID-19 as a risk on the consent form should be obtained. This is important for the transition period.
- Liaise with the infection control team on the best mechanism for donning and doffing, if deemed appropriate.
- All healthcare professionals should undertake frequent hand washing. Reviewing the literature, normal soap might be all that is required. The hand washing technique should be stressed upon and each episode should last at least 20 seconds. The temperature of the water is less important than the amount of time that is spent washing hands32,51.
- Several adaptations to our daily habits have to be made following this pandemic. These changes may be implemented into our future routine. These include measures such as avoidance of touching our face with our hands and some degree of social distancing can be of value for considerable time. We therefore recommend adherence to social distancing guidelines51.
- Healthcare professionals are advised to wear masks whilst in the operating theatre at all times.
Implementing multidisciplinary team meeting
Throughout the transition back to elective work, it is advised for a multidisciplinary team (MDT) meeting to be implemented33. This should include surgeons, anaesthetists, management staff and theatre staff. It is imperative for these meetings to happen daily through the initial transition phases to discuss several pivotal issues, including PPE, pandemic assessment, patient backlog, safety and quality. Through phase II and phase III the MDT should meet weekly to ensure the transition is streamlined providing patients with the most effective care. The MDT should also review the quality of care metrics including readmission rates, complications and morbidity and mortality rates.
Summary
In summary, the guidance for a three-phase process for return to elective orthopaedic work in the most effective and efficient manner. This is evidence based guidance and can be implemented taking the local hospital needs into consideration. It is important throughout the transitions back to elective work, all senior health care professionals are responsive and supportive to patients and staff. It is imperative to provide all patients with respect, compassion and dignity whilst continuing to improve quality of care.
Conflicts of Interest
The authors of this publication have no conflicts of interests to declare with regards to this work.
Funding Statement
This publication received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- He F, Deng Y, Li W. Coronavirus Disease 2019 (COVID‐19): What we know? J Med Virol. 2020 Mar 14. [Epub ahead of print].
- Hunter P. The spread of the COVID-19 coronavirus: Health agencies worldwide prepare for the seemingly inevitability of the COVID-19 coronavirus becoming endemic. EMBO Rep. 2020;21(4):e50334.
- Hassan S, Sheikh F, Jamal S, Ezeh J, Akhtar A. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cureus. 2020;12(3):e7355.
- PAHO (2020). PAHO Director's remarks on COVID-19 pandemic - 11 March 2020. Available at: https://www.paho.org/en/documents/paho-directors-remarks-covid-19-pandemic-11-march-2020.
- World Health Organization (2020). WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- Worldometers.info (2020). Coronavirus Update (Live): 2,987,043 Cases and 206,679 Deaths from COVID-19 Virus Pandemic. [cited 26 April 2020]. Available at: https://www.worldometers.info/coronavirus.
- European Centre for Disease Prevention and Control (2020). Disease background of COVID-19. Available at: https://www.ecdc.europa.eu/en/2019-ncov-background-disease.
- Guan W, Liang W, Zhao Y, Liang H, Chen Z, Li Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020 Mar 26. [Epub ahead of print].
- Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 Mar 31;368:m1295.
- Livescience (2020). Treatments for COVID-19: Drugs being tested against the coronavirus. Available at: https://www.livescience.com/coronavirus-covid-19-treatments.html.
- Lythgoe M, Middleton P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol Sci. 2020 Apr 9. [Epub ahead of print].
- Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225-8.
- Hollander JE, Carr BG. Virtually Perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-81.
- Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill. 2020 Mar;25(12).
- British Orthopaedic Association (2020). Information for BOA members on trauma and orthopaedic care in the UK during coronavirus pandemic. Available at: https://www.boa.ac.uk/resources/statement-for-boa-members-on-trauma-and-orthopaedic-care-in-the-uk-during-coronavirus-pandemic.html.
- NHS England (2020). Available at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/20200317-NHS-COVID-letter-FINAL.pdf
- Ilman J. Exclusive: NHS prepares to cancel elective ops in readiness for covid-19 surge. Available at: https://www.hsj.co.uk/free-for-non-subscribers/exclusive-nhs-prepares-to-cancel-elective-ops-in-readiness-for-covid-19-surge/7027110.article.
- CONGRESS.GOV (2020). Congress 116th. S.3548 - CARES Act. Available at: https://www.congress.gov/bill/116thcongress/senate-bill/3548/text.
- US Small Business Administration (2020). Coronavirus (COVID-19): small business guidance & loan resources. Available at: https://www.sba.gov/page/coronavirus-covid-19-small-business-guidance-loan-resources.
- Anoushiravani AA, O’Connor CM, Dicaprio M, Iorio R. Economic impacts of the COVID-19 crisis an orthopaedic perspective. J Bone Joint Surg Am. 2020:1e9. [Epub ahead of print].
- Coronavirus (COVID-19) cases in the UK. [cited 26 April 2020]. Available at: https://coronavirus.data.gov.uk/#regions.
- American Enterprise Institute (2020). National coronavirus response: A road map to reopening. Available at: https://www.aei.org/research-products/report/national-coronavirus-response-a-road-map-to-reopening.
- https://www.wsj.com/podcasts/the-journal.
- Institute for Health Metrics and Evaluation (2020). Social distancing assumed until infections minimized and containment implemented. Available at: https://covid19.healthdata.org/united-states-of-america/illinois.
- O’Connor C, Anoushiravani A, DiCaprio M, Healy W, Iorio R. Economic Recovery Following the COVID-19 Pandemic: Resuming Elective Orthopaedic Surgery and Total Joint Arthroplasty. J Arthroplasty. 2020 Apr 18. [Epub ahead of print]
- Irio R, Davis CM, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery. A survey of the american association of hip and knee surgeons. J Arthroplasty. 2010;25(7):1005-14.
- Mitchell G. [Nursing Times]. Deaths from all causes in care homes double over course of pandemic. Available at: https://www.nursingtimes.net/news/coronavirus/deaths-from-all-causes-in-care-homes-double-over-course-of-pandemic-21-04-2020.
- Bialek S, Boundy E, Bowen V, Chow N, Cohn A, Dowling N, et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-6.
- McMichael T, Currie D, Clark S, Pogosjans S, Kay M, Schwartz N, et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020 Mar 27. [Epub ahead of print].
- Zimmerman S, Sloane P, Katz P, Kunze M, O'Neil K, Resnick B. The Need to Include Assisted Living in Responding to the COVID-19 Pandemic. JAMDA. 2020;21(5):572-5.
- Centers for Disease Control and Prevention (2020). Standard Operating Procedure (SOP) for Triage of Suspected COVID-19 Patients in non-US Healthcare Settings: Early Identification and Prevention of Transmission during Triage. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/sop-triage-prevent-transmission.html.
- Russell A. [Global News]. Coronavirus: When will a COVID-19 vaccine be ready? Available at: https://globalnews.ca/news/6666876/coronavirus-covid-19-vaccine.
-
Vannabouathong C, Devji T, Ekhtiari S, Chang Y, Phillips S, Zhu M, et al. Novel Coronavirus COVID-19: Current Evidence and Evolving Strategies. J Bone Joint Surg Am. 2020 Apr 1. [Epub ahead of print].
- American Association of Orthopaedic Surgeons (2020). Navigating the COVID-19 Pandemic. Available at: https://www.aaos.org/globalassets/about/covid-19/aaos-clinical-considerations-during-covid-19.pdf.
- Association of periOperative Registered Nurses (2020). Joint Statement: Roadmap for Resuming Elective Surgery after COVID-19 Pandemic. Available at: https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19.
- NHS Foundation Trust - Dorset County Hospital (2020). Infection Prevention & Control: MSSA Treatment Guidance Octenisan. Available at: https://www.dchft.nhs.uk/patients/patient-information-leaflets/Documents/MSSA%20Patient%20Leaflet%20(April%202019).pdf.
- NHS Foundation Trust - Sheffield Teaching Hospitals (2020). Antiseptic washes to reduce MSSA carriage. Available at: https://publicdocuments.sth.nhs.uk/pil3887.pdf.
- NHS Foundation Trust - Northumbria Healthcare (2020). MSSA screening for pre-assessment patients. Available at: http://api.gp.northumbria.nhs.uk/uploads/patient_info/Infection%20Control/MSSA_screening_pre-assessment_patients.pdf.
- Jeans E, Holleyman R, Tate D, Reed M, Malviya A. Methicillin sensitive staphylococcus aureus screening and decolonisation in elective hip and knee arthroplasty. J Infect. 2018;77(5):405-9.
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020 Mar 10. [Epub ahead of print].
- World Health Organization (2020). Coronavirus disease 2019 (COVID-19) Situation Report – 73. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf.
- Virlogeux V, Fang VJ, Park M, Wu JT, Cowling BJ. Comparison of incubation period distribution of human infections with MERS-CoV in South Korea and Saudi Arabia. Sci Rep. 2016;6:35839.
- Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291-300.
- Tingle J. Patient safety and litigation in the NHS post-COVID-19. Br J Nurs. 2020;29(7):444-5.
- Elston D. The coronavirus (COVID-19) epidemic and patient safety. J Am Acad Dermatol. 2020;82(4):819-20.
- Schwartz J, King C, Yen M. Protecting Healthcare Workers During the Coronavirus Disease 2019 (COVID-19) Outbreak: Lessons From Taiwan’s Severe Acute Respiratory Syndrome Response. Clin Infect Dis. 2020 Mar 12. [Epub ahead of print].
- The Lancet. COVID-19: protecting health-care workers. Lancet. 2020 Mar;395(10228):922.
- Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020 Mar 6. [Epub ahead of print].
- Horton R. Offline: COVID-19 and the NHS—“a national scandal”. Lancet. 2020;395(10229):1022.
- Dailiana Z, Papakostidou I, Varitimidis S, Liaropoulos L, Zintzaras E, Karachalios T, et al. Patient-reported quality of life after primary major joint arthroplasty: a prospective comparison of hip and knee arthroplasty. BMC Musculoskelet Disord. 2015;16:366.
- Yang C. Does hand hygiene reduce SARS-CoV-2 transmission? Graefes Arch Clin Exp Ophthalmol. 2020;258(5):1133-4.
