A practical approach to artificial intelligence in trauma and orthopaedics
Authors: Andrew Coppola and Vipin Asopa
A practical approach to AI in T&O
Andrew Coppola is an Orthopaedic Research Fellow at the South West Elective Orthopaedic Centre, Epsom. He holds an MSc in Surgical Innovation from Imperial College, where he focused on predictive artificial intelligence in knee and hip replacements. He has been awarded an NHS Topol Digital Fellowship to advance transformative artificial intelligence applications in orthopaedics.
Vipin Asopa is a Specialist Hip and Knee Surgeon at South West London Elective Orthopaedic Centre, Epsom. His research interests include the use of artificial intelligence to improve patient outcomes following surgery.
The concept of artificial intelligence (AI) first emerged in the 1940s. Progress was slow with researchers facing numerous challenges over the years. It was not until the late 1990s and early 2000s, that real-world applications started to appear.
Examples of this include recognition of handwritten postcodes (AT&T, US postal service) and robot pets. The 2010s saw a big leap, with the release of Microsoft’s Kinect, which was able to recognise human gestures and Apple’s voice recognition tool, Siri. A breakthrough occurred in 2012, when Hinton won ImageNet’s image recognition competition, that AI and deep learning was popularised1. His work has formed the basis of computer vision (the application of deep learning to image and videographer recognition) and contributed to the development of large language models that have revolutionised the potential for analysis of unstructured text data.
Although AI is a hot topic in trauma and orthopaedics, commercial applications remain limited. One example is the Sectra Amplifier marketplace AI platform, is BoneView (Gleamer AI), which assists junior doctors in detecting fractures2. Another example is OrthoSensor’s VERASENSE™ technology, which utilises AI to enhance implant positioning during joint replacement surgery3. Interest in the application of AI techniques to managing musculoskeletal problems has grown over the last couple of years due to the realisation that there is a large amount of data available in and outside of hospitals (such as registries). At the 2023 AI in Orthopaedics meeting, hosted by ORUK, several abstracts were presented covering the application of three main AI/ML disciplines: large language models, computer vision and predictive analytics (identify patterns from past data to predict future events). Since this meeting, numerous projects in these areas are nearing completion and publication or commercialisation.
Examples of the use of large language model (natural language processing) AI to make clinical decisions include making clinical decisions based on radiology reports4 and testing whether it is clever enough to pass the FRCS exam (Turing stated in 1940, that for true intelligence, a machine needs to provide answers to questions that are indistinguishable from human responses)5.
Computer vision has been used to produce 3D images from 2D radiographs and to detect and classify fractures6,7. Others have studied quality assurance through the analysis of post-operative radiographs and used image analysis to predict outcomes following joint surgery [personal communication]. Although still at research stage, these papers represent the fast pace at which AI is developing in the trauma and orthopaedic space.
Predictive analytics uses statistical techniques and machine learning to forecast outcomes from historical data. In trauma and orthopaedics, it has been used to predict the duration of surgical procedures in the hope of improving operating theatre scheduling.
The application of AI techniques to clinical problems can include using a variety of techniques such as simple equations to complex deep learning methods (a subset of machine learning). Deep learning is viewed as a black-box because it is not known how the algorithm works although computational techniques can explain some ability of models7,8. Generative AI can be used to create intelligible content from diverse data, for example summarising research publications and clinic letters9.
Figure 1: Artificial Intelligence disciplines. This figure illustrates the three main disciplines within AI: Predictive Analytics, Natural Language Processors, and Computer Vision. Each discipline represents a distinct area of AI applications.
AI algorithms are developed through training and testing. The broad method of AI applied depends on the question and answer being sought and the data available. For example, computer vision is based on the use of images for analysis. Natural language processing, or handling text is often carried out using large language models. In setting up an AI project, it is important to consider how data will be obtained – for example downloading radiographic images from consented patients. Training requires the use of non-bias data, sometimes using the technique of optimisation and feature selection. Reporting on algorithms should be transparent (e.g. using the PROBAST and CHARMS frameworks)10. The deployment of a model and how its function will be verified needs to be planned.
The practical considerations of setting up an AI project in an NHS hospital include defining a research project, obtaining ethics / research approval (if indicated), discussing the project with the Caldicott Guardian, obtaining host organisation support and finally, patient consent if needed. AI algorithms can be developed and run on a virtual machine (hosted in the cloud on Microsoft Azure or Amazon Web Services) or a physical computer located within the hospital environment. Cloud machines may be easier to manage, simpler to upscale and do not require physical support services, however they can be costly. Both require consideration of the resources: a powerful central processing, graphics processing unit and fast storage and large memory capacity. Data security is worthy of consideration along with remote access (VPN) for researchers.
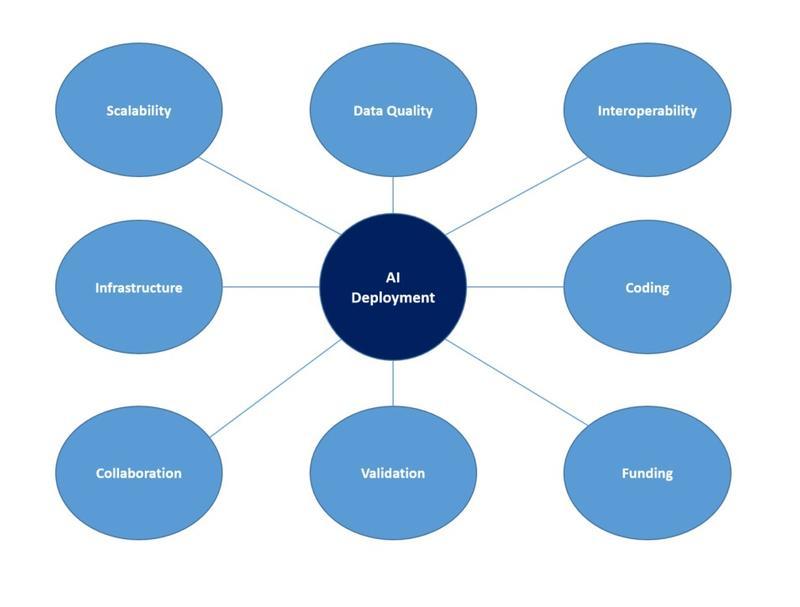
Figure 2: Practicalities of Artificial Intelligence. This figure shows eight key considerations for deploying AI.
Using and developing AI tools may require an understanding of coding i.e. Python. Examples of computer code are available online. This can be complex and a team-based approach may be appropriate involving university collaboration and/or a data analysist. Once developed, the model will require external validation to assess generalisability.
Governance matters need to be considered. Is consent required to use patient data? Using anonymised data as part of a quality improvement project may negate, but advice should be obtained from research management or the HRA. The Caldicott Guardian should be consulted to ensure patient data is used appropriately.
Access to different data sources is challenging. There is a requirement to improve connectivity between different data sources and software applications within and outside of the hospital environment. Improved connectivity will reduce the need for manual downloading of data and improve the accuracy of algorithms by reducing digital divides13. Standardised APIs to allow connectivity and sharing should be developed instead of aggregating data into large repositories under the control of one company.
Funding for projects may be a concern, but there are organisations that provide funding for research projects relating to musculoskeletal conditions including the Gwen Fish Trust and ORUK (Orthopaedic Research UK). Recent initiatives by the government are likely to increase the amount of money available for AI research14.
MHRA approval is required before a working AI tool can be safely used in clinical practice. They will only issue a UKCA certificate once the algorithm has gone through extensive testing. Changes to the algorithm after a certificate has been issued will require new authorisation. As a result, there is currently no mechanism for algorithms to continually learn and update themselves with new information11,12.
Commercialisation is the step required to obtain large amounts of funding to take promising research projects through further development including obtaining regulatory approval. However, various questions related to intellectual property need to be considered including who does the algorithm belong to when such a product is brought to market? Do the developers get a share? Does the NHS, who provide the data source, control all rights?
Many of the topics in this article will be discussed at the BOA-supported AI in Orthopaedics meeting to be held 19th - 20th December 2024 at the Royal College of Surgeons of England and we look forward to welcoming you at this event!
References
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Commun ACM. 2017;60:84-90.
- Oppenheimer J, Lüken S, Hamm B, Niehues SM. A Prospective Approach to Integration of AI Fracture Detection Software in Radiographs into Clinical Workflow. Life. 2023;13(1).
- Al-Nasser S, Noroozi S, Harvey A, et al. Exploring the Performance of an Artificial Intelligence-Based Load Sensor for Total Knee Replacements. Sensors. 2024;24(2).
- Li MD, Deng F, Chang K, et al. Automated Radiology-Arthroscopy Correlation of Knee Meniscal Tears Using Natural Language Processing Algorithms. Acad Radiol. 2022;29:479-87.
- Turing A. Computing Machinery and Intelligence. Mind. 1950;49:433-60.
- Lind A, Akbarian E, Olsson S, et al. Artificial intelligence for the classification of fractures around the knee in adults according to the 2018 AO/OTA classification system. PLoS One. 2021;16:e0248809.
- Olczak J, Emilson F, Razavian A, et al. Ankle fracture classification using deep learning: automating detailed AO Foundation/Orthopedic Trauma Association (AO/OTA) 2018 malleolar fracture identification reaches a high degree of correct classification. Acta Orthop. 2020;92:102-8.
- Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-44.
- Buhrmester V, Münch D, Arens M. Machine learning & knowledge extraction Analysis of Explainers of Black Box Deep Neural Networks for Computer Vision: A Survey. 2021:arXiv:1911.12116v1 doi.org/10.48550/arXiv.1911.12116.
- Collins GS, Dhiman P, Andaur Navarro CL, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 2021;11:e048008.
- Chen JH, Alagappan M, Goldstein MK, et al. Decaying relevance of clinical data towards future decisions in data-driven inpatient clinical order sets. Int J Med Inform. 2017;102:71-9.
- Software and Artificial Intelligence (AI) as a Medical Device - GOV.UK. Available at: www.gov.uk/government/publications/software-and-artificial-intelligence-ai-as-a-medical-device/software-and-artificial-intelligence-ai-as-a-medical-device. Accessed 2 April 2024.
- New £100 million fund to capitalise on AI’s game-changing potential in life sciences and healthcare - GOV.UK. Available at: www.gov.uk/government/news/new-100-million-fund-to-capitalise-on-ais-game-changing-potential-in-life-sciences-and-healthcare. Accessed 2 April 2024.
- Sagi A, Asopa V, Mitchell M, et al. The digital divide between primary and secondary care: An analysis using SARS-CoV-2 hospital admissions. Health Informatics Journal. 2024;30(3).
